Mycoplasma pneumoniae
Mycoplasmas are atypical bacteria that live as intra- or extracellular parasites. Mycoplasmas can be transmitted through aerosols (M. pneumoniae, M. fermentans, both of which are found in the saliva); sexual transmission is also frequent (M. genitalium). Acute M. pneumoniae infections cause pneumonia, bronchitis; chronic infection in the lungs can exacerbate other respiratory diseases such as asthma. Mycoplasmas can disseminate from their primary infection site to other organs. Central nervous system can be a target, resulting in encephalitis; attack on the joints and development of arthritis is also frequent.
Mycoplasma infections are particularly frequent in CFS customers. Using PCR detection, M. fermentans was found in 34% of CFS customers, versus 8% of healthy controls. Another study showed that more than two third of CFS customers (versus 5.6% of controls) were infected by at least one mycoplasma species (M. fermentans, M. pneumoniae, or M. hominis).
This high prevalence may result from the immunodepression typically observed in CFS (low NK activity); however persistent mycoplasma infections can in turn contribute to the etiology of the disease by eliciting a chronic inflammatory response.
Chlamydia trachomatis and chlamydia pneumoniae
Chlamydiae are bacterial intracellular pathogens which cause widespread infections in humans.
C. trachomatis is the world’s most common sexually transmitted bacterial pathogen. C. pneumoniae, which is transmitted via respiratory secretions, causes pneumonia.
The percentage of people displaying positive serology to C. pneumoniae is high, reaching 80% in adults.
Chlamydial organisms have the capacity to enter a particular growth stage characterized by reticulate bodies that divide very slowly and can persist in cells for a long time. This results in a chronic infection, which, by inducing a sustained inflammatory response, can lead to a number of serious pathologies.

Chlamydia pneumoniae
Acute C. pneumoniae infections cause pneumonitis; chronic persistence of the pathogen in the lungs has been linked to chronic obstructive pulmonary disease, asthma, and even lung cancer. In the lungs, C. pneumoniae can infect alveolar macrophages and spread to other organs via the blood. Infection can be directly transferred to vascular endothelial cells, leading to chronic endothelium inflammation that favors atherogenesis. Chlamydia infection stimulates the migration of monocytes through the blood-brain barrier, promoting inflammation of the central nervous system. An increased prevalence of C. pneumoniae infections has been reported in CFS customers.
Chlamydia trachomatis
C. trachomatis has a tropism for both conjunctival and urogenital epithelial cells. Ocular infections cause conjunctivitis, that frequently evolve to trachoma. Urogenital infections cause acute urethritis. Like C. pneumoniae, C. trachomatis can disseminate away from the site of primary infection. Several days after a genital infection, certain customers develop acute inflammatory arthritis; this is caused by C. trachomatis organisms that have reached the joint via circulating monocytes. A portion of these customers will then develop chronic arthritic disease. Persistent, chronic chlamydial infections may have little or no apparent symptoms. Though, they continue to elicit a chronic inflammation that will eventually cause disease. This of course strongly warrants screening for infections. Monocytes appear as common host cells for persistent organisms, and major effectors of systemic dissemination. PCR testing in whole blood is therefore an adequate approach for the detection of Chlamydia infections.
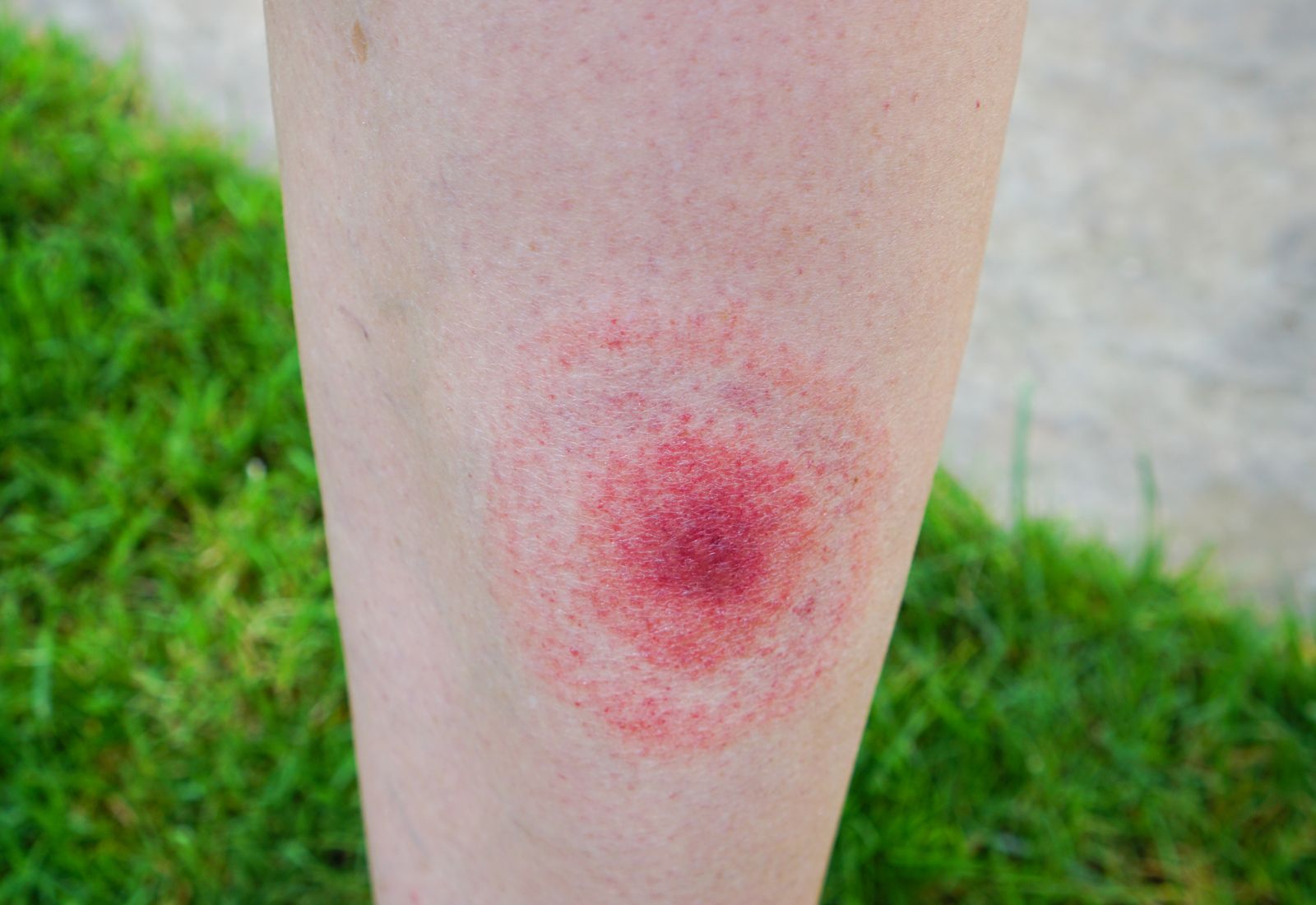
Borrelia
Borreliosis is a worldwide infectious disease caused by spiral-shaped bacteria known as Borreliae, carried by ticks and louse. Borrelia is a genus of bacteria of the spirochete phylum.
Borrelia is divided into two main clades, the Borrelia burgdorferi sensu lato group and the relapsing fever group.
The B. burgdorferi sensu lato group contains 20 species (like B. burgdorferi,B. afzelii, B. garinii, B. Spielmanii, B. bavariensis, etc), including the causative agents of Lyme borreliosis, and are solely transmitted by hard-bodied ticks. Lyme disease (LD) is the most common tick-born disease with approximately 476,000 customers in the United States annually during 2010–2018 (Kugeler et al., 2021). The LD-causing bacteria are generally transmitted to humans after they are bitten by ticks of the Ixodes family infected with LD causing Borrelia. However, recent reports have raised concerns over Borrelia transmission through blood transfusion based on observations that Borrelia can survive and circulate in the human bloodstream (Pavia and Plummer, 2018).
Lyme disease exhibits a variety of symptoms that may be confused with immune and inflammatory disorders. Inflammation around the tick bite causes skin lesions. Erythema (chronicum) migrans (ECM), a unique expanding skin lesion with central clearing that has a ring-like appearance, is typically the first stage of the disease. Arthritis, neurological disease, and cardiac disease may be later stage manifestations. For signs and symptoms, refer to https://www.cdc.gov/lyme/signs_symptoms/index.html
The relapsing fever group consists of 25 species that includes Borrelia miyamotoi, B. recurrentis, B. hermsii, B. duttonnii, etc. Most of the relapsing fever species are transmitted by soft-bodied ticks but some species are transmitted by hard-bodied ticks (B. miyamotoi, Borrelia lonestari, Borrelia theileri) or by lice (Borrelia recurrentis). B. miyamotoi shares phenotypic characteristics of the relapsing fever group such as relapsing fever, a high level of spirochetemia in blood, and transovarial transmission; however, it also has some characteristics of the B. burgdorferi sensu lato group, most notably transmission by hard-bodied ticks. In contrast to Lyme disease, the rash was uncommon, with fewer than 1 in 10 customers developing a rash.
RF Borrelia are reported to develop immune evasion strategies. One prominent strategy developed by RFB to evade innate immunity involves inactivation of complement by recruiting distinct complement regulatory proteins. In addition, RFB possess a unique system of antigenic variation, allowing them to change the composition of surface-exposed variable major proteins, thus evading the acquired immune response of the human host ( see https://www.frontiersin.org/articles/10.3389/fimmu.2020.01560/full ).


Other tick-borne infections
Borreliosis is the most known tick-borne infection, but it is not the only one, many other pathogens are transmitted by the ticks with or without Borrelia. Clinically relevant tick-borne infections (previously named "co-infections") are caused by Babesia, Bartonella species, Rickettsia, Anaplasma, Ehrlichia, several viruses (like Heartland virus, Powassan virus, Bourbon virus, Tick-borne encephalitis (TBE) virus, ) etc. Infections caused by these pathogens in customers not infected by Borrelia burgdorferi can result in clinical symptoms similar to those occurring in Lyme disease.
Chlamydia trachomatis primarily causes polyarthritis. Chlamydophila pneumoniae not only causes arthritis but also affects the nervous system and the heart, which renders the differential testing solution difficult. The testing solution is even more complex when co-infections occur in association with Lyme disease. (from Berghoff W. Open Neurol J. 2012;6:158-78.)